2020-06-01 by Quick Biology Inc.
Quick Biology has been posting lots of eukaryotic single cell profiling methods and their applications in biomedical, stem cell field (Fig. 1, ref1). Bacterial cells, however, have lagged a lot due to the presence of thick prokaryotic cell (lysis challenge) and lack of poly(A) tails mRNA (capture efficiency in reverse transcription).
In current Nature Microbiology, Scientists in Columbia University borrow two concepts i) single cell combinatorial indexing ii) cells are themselves as compartments for barcoding (ref2), developed prokaryotic expression-profiling by tagging RNA in situ and sequencing (PETRI-seq) (Fig. 2). As shown in Fig.2, they use combinatorial cDNA barcoding within cells themselves, individual transcripts are specifically labeled by three rounds of barcoding, in each round, pooled cells are randomly distributed into different cells. Through NGS reads decoding barcodes and UMIs (Unique molecule Index), researches can track where and which cell type the reads come from. PETRI-seq, unlike Drop-seq, Seq-well, does not require complex cell partitioning instruments, which make it simple and affordable. Finally, scientist tested PETRI-seq performance using species-mixing experiments, PETRI-seq successfully discovers single-cell states, and dynamics in complex microbial communities (Fig.3).
Figure 1: Timeline of sing cell RNA-seq method development (adapted from ref1). Timeline of key developments in eukaryotic and prokaryotic scRNA-seq detailing the number of cells sequenced in each experiment. Prokaryotic scRNA-seq has lagged significantly behind eukaryotic scRNA-seq due to technical challenges. In 2011, the first single-cell microarray study by Kang et al was described for a few Burkholderia thailandensis cells, each containing 2 pg of RNA, orders of magnitude more than many bacterial species of interest. More recent reports described the sequencing of six Synechocystis sp. PCC6803 cells and three Porphyromonas somerae cells, each of which contains 1-5 fg of RNA. These methods comprehensively characterized the transcriptomes of a few single cells. However, they are prone to contamination and not equipped to study highly heterogeneous bacterial communities and rare populations like persisters across thousands of cells.
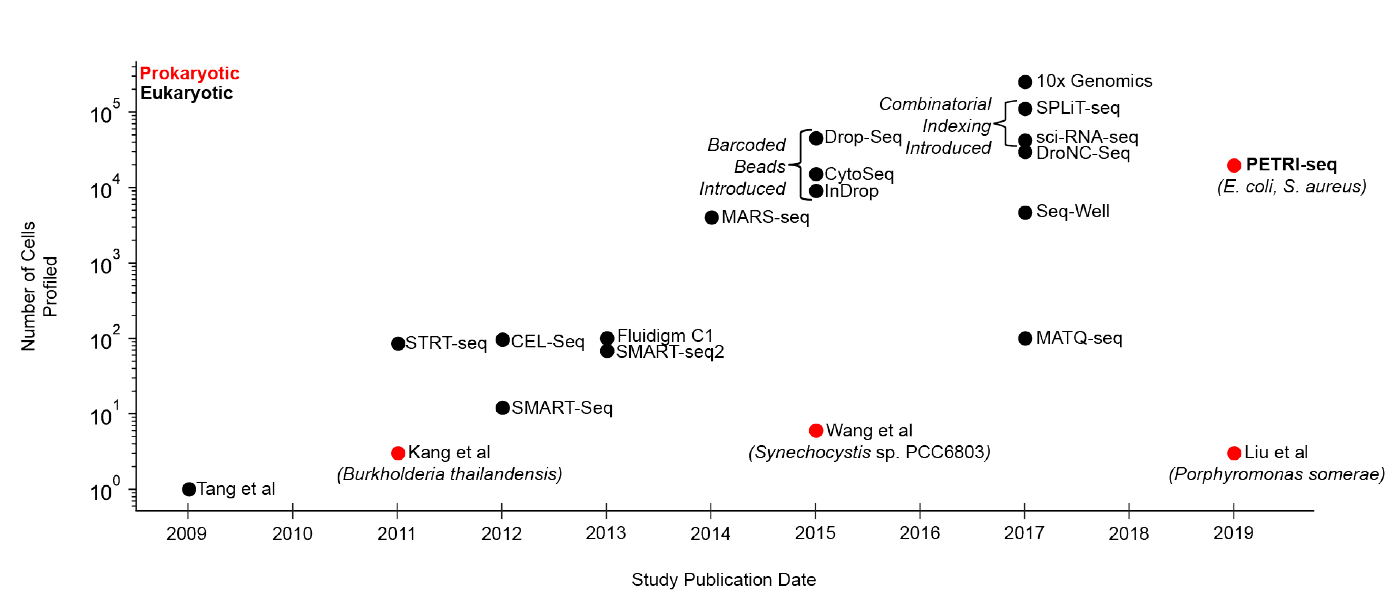
Figure 2: Overview of PETRI-seq (adapted from ref1). PETRI-seq includes three parts: cell preparation, split-pool barcoding, and library preparation. In cell preparation, cells are prepared for in situ reactions by fixation (formaldehyde) and permeabilization (lysozyme/lysostaphin). During split-pool barcoding, cells are split across 96- well plates three times for three rounds of barcoding by reverse transcription and two ligations. After barcoding, cells are lysed to release cDNA, which is subsequently prepared for paired-end Illumina sequencing. Each cDNA fragment in the library includes a unique molecular identifier (UMI) and 3 barcodes, which are all sequenced in Read 1. The UMI is a sequence of 7 degenerate nucleotides that can distinguish unique transcripts from PCR duplicates. The 3 barcodes comprise a barcode combination (BC), which allows reads to be grouped by their cell of origin. In Read 2, the cDNA is sequenced.
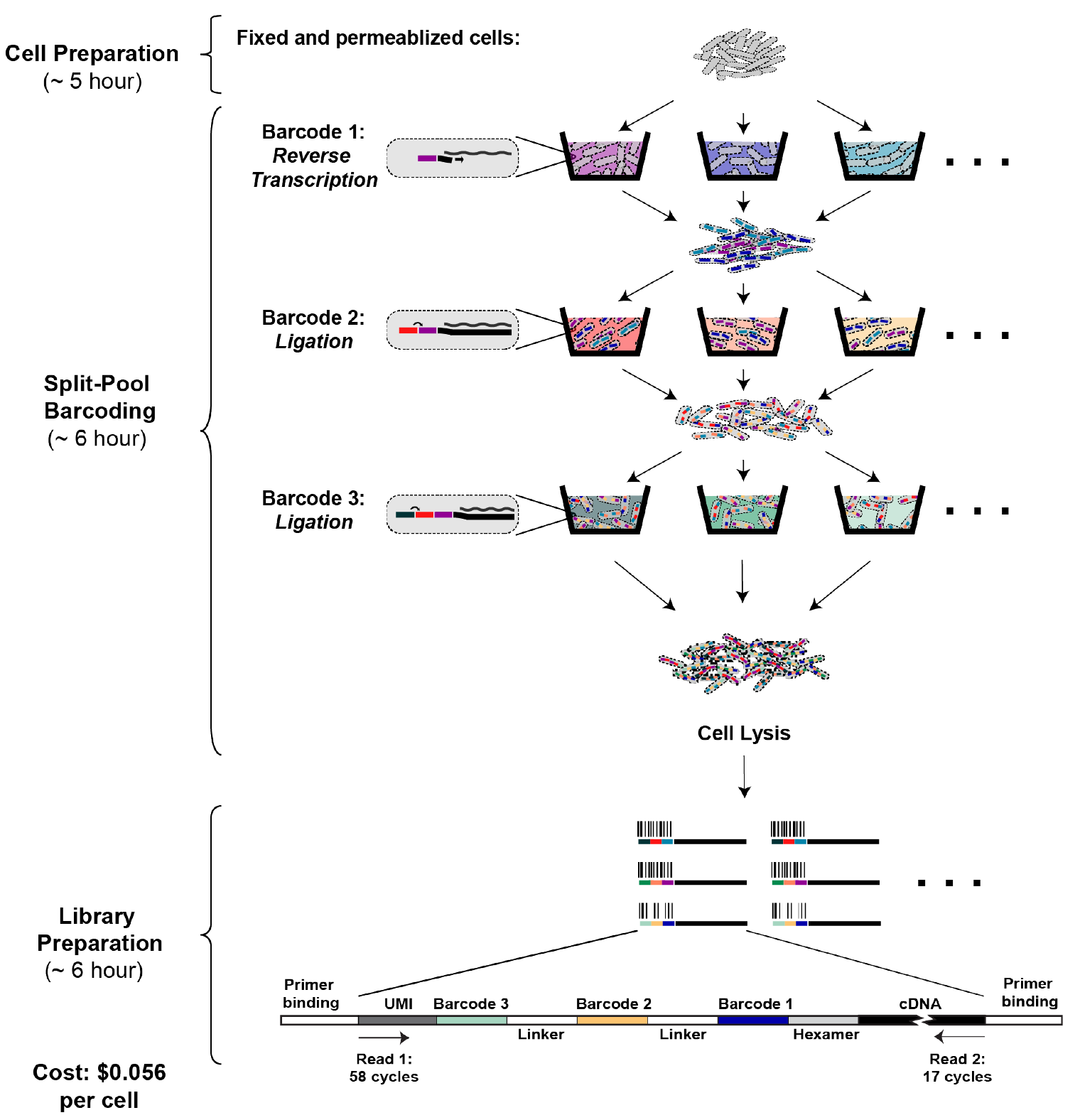
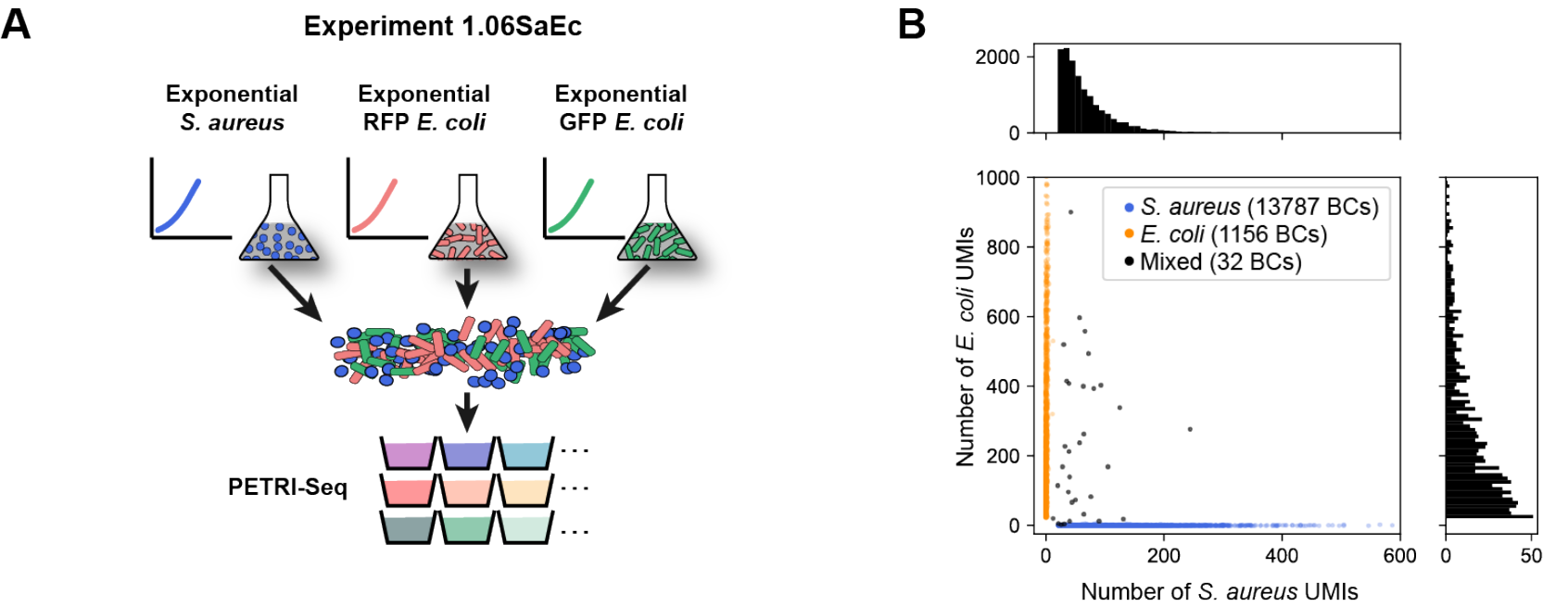
Figure 3: Species mixing experiments for validation of PETRI-seq. (A) S. aureus and E. coli cells were grown separately then mixed for PETRI-seq after cell preparation. (B) Species mixing plot for E. coli and S. aureus based on total UMIs per BC, including rRNA. BCs were assigned to a single species if more than 90% of UMIs mapped to that species and fewer than 20 UMIs mapped to the other species. Histograms (top, right), show the number of S. aureus or E. coli cells (respectively) with the corresponding number of total UMIs.
Quick Biology is an expert in NGS assay development. Find More at Quick Biology.
Ref:
- 1. Blattman S, Jiang W, Oikonomou P and Tavazoie S. Prokaryotic single-cell RNA sequencing by in situ combinatiorial indexing. Nature Microbiology (2020, Published 25 May 2020) https://www.nature.com/articles/s41564-020-0729-6
- 2. https://www.splitbiosciences.com/technology